✕
✕
For Patients & Families
Our Science
About Us
Connect with Us

Our lead program candidate, navenibart (STAR-0215), is a potential best-in-class monoclonal antibody inhibitor of plasma kallikrein designed to provide potential long-acting, safe, and effective attack prevention for hereditary angioedema (HAE), with the potential for dosing every 3 and 6 months. The prefix “nav-” represents Astria’s commitment to navigating the development of navenibart with patients as our guiding stars. The infix “-eni-” represents “enzyme inhibitor” and the suffix “-bart” represents “monoclonal antibody.”

Learn how navenibart aims to prevent attacks in HAE by exploring the mechanism of action here:
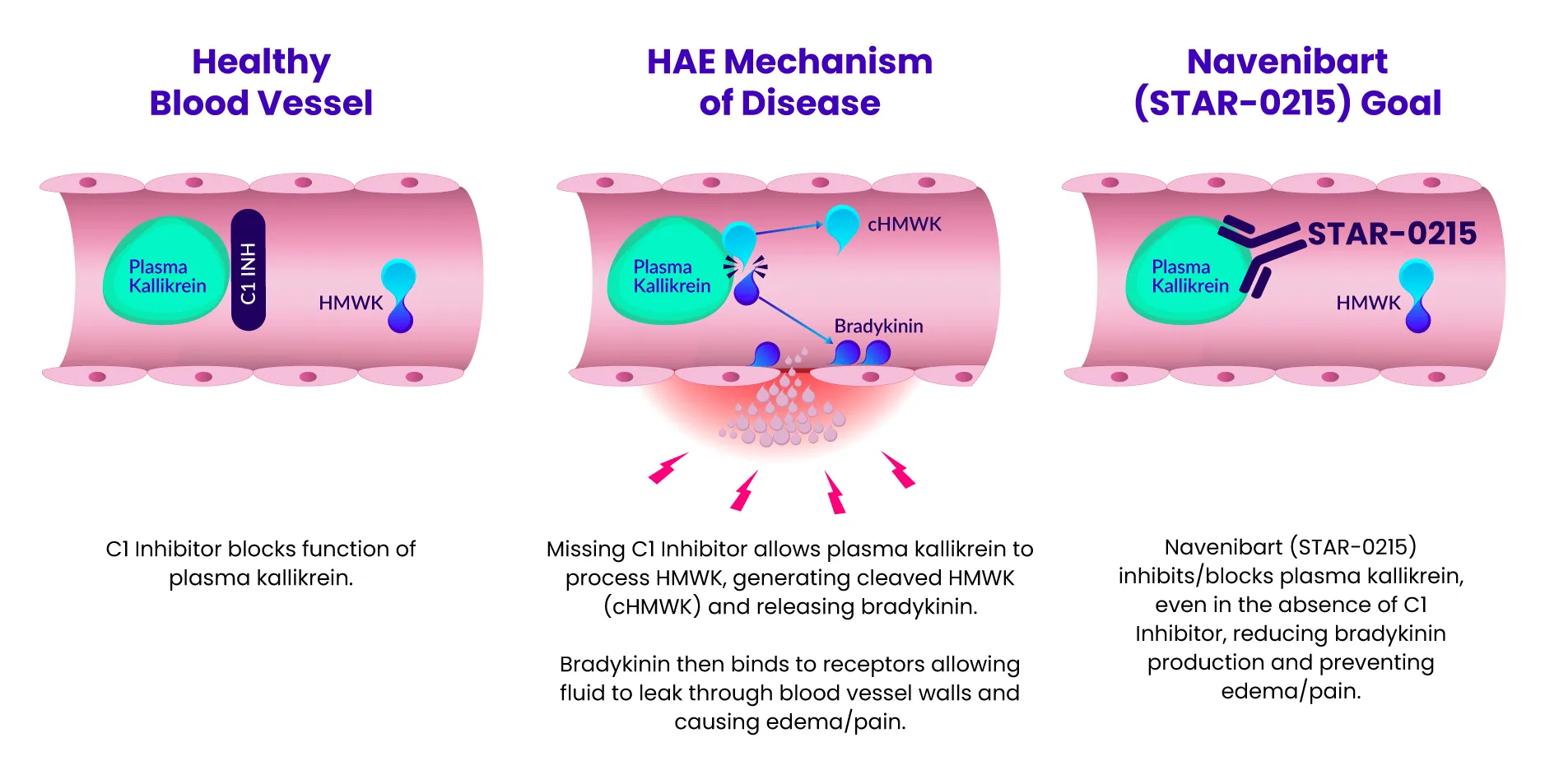

We’ve heard from the HAE community the desire to normalize their lives, living without limitations from their symptoms and treatments. Our goal is to reduce the disease and treatment burden for people living with HAE, whose current options for preventative therapies require frequent administration (daily or up to every 4 weeks). We believe we can do better with navenibart.
Based on the near-term HAE treatment landscape, positive results from target enrollment in the Phase 1b/2 trial support our vision that navenibart can become the market-leading HAE treatment with dosing every 3 and 6 months.